Packaging
OYSTAR Hüttlin: Cleaning Validation Workshop
Wednesday 22. October 2008 - Leveraging the Knowledge Base
A highly skilled workforce is crucial to the success of any business. This is particularly true of the high-tech industry, which includes some pharmaceutical companies. Awareness of this fact has prompted OYSTAR Hüttlin, a member of the OYSTAR Group, to schedule a series of educational seminars for pharmaceutical industry employees to enable them to obtain certification as Expert of Solids and Master of Solids. The workshops will begin with the most important process after the actual manufacturing process: The cleaning process and its validation.
In recent years OYSTAR Hüttlin has evolved from a supplier of equipment into a provider of integrated value chain solutions for the production of solid pharmaceuticals. The OYSTAR-Hüttlin pharmaceutical service has played a major role in this process – only last year, the company invested around 6 million in this service at its new Schopfheim facilities. The newly constructed and equipped pilot laboratories offer a comprehensive range of services covering formulation and process development, scale-up processes, process optimization, troubleshooting, and PAT consulting.
Training programs for customer employees are a key service offering. In response to customer requests, OYSTAR Hüttlin has designed two sets of consecutive seminars. The company will use these workshops to communicate the solids manufacturing know-how that it has built up over decades and to present new technologies.
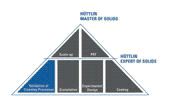
Customer employees can qualify as an Expert of Solids in four seminars that teach skills relating to the development and production of solids, cleaning, and validation. These workshops will enable participants to acquire specialized knowledge in the following fields: granulation process, influence of granules on tabletting, solids coating, experimental developmental design, and process optimization.
Customer employees can obtain the qualification of Hüttlin Master of Solids by attending two further seminars in which they will learn about the production of different types of solids in greater detail. The material taught includes Process Analytical Technology (PAT) criteria and scale-up processes.
The series of international workshops begins in November with a seminar on cleaning process validation. This is an extension of the new clean-in-place solution featuring a metal filter that opens downwards, a new process that is currently being patented. Speakers come from Ecolab, F. Hoffmann-La Roche, OYSTAR Hüttlin and TechPharm and will give in-depth insights into the cleaning process and validation methods. After the course, the participants will be able to prepare Standard Operating Procedures (SOP) for the cleaning process and perform cleaning validation. The workshops are limited in size so that each attendee can receive in-depth, individual attention.
